
Because we can safely and effectively perform this procedure, we can perform work on extremely delicate areas. Ultrasonic waves are used in the treatment of the brain to generate a distinct thermal lesions without causing damage to surrounding structures. By focusing ultrasound on a specific region, it can induce an ablation in the same area that causes tremor and Parkinsons symptoms. Halpern, the departments chief of Stereotactic and Functional Neurosurgery, is a physician at The University of Pennsylvania. This medication is intended to reduce dyskinesia in Parkinsons disease patients. MRI-guided focused ultrasound is an option for treating patients at Penn Medicine. This means that medicare does not cover this treatment. There is no mention of focused ultrasound for parkinsons disease in the medicare coverage documents. Recommended Reading: Cognitive Impairment In Parkinson’s Disease Does Medicare Cover Focused Ultrasound For Parkinsons Disease The MRI scanner provides images for the physician to clearly see the treatment area and provides images that show changes in temperature at the target.

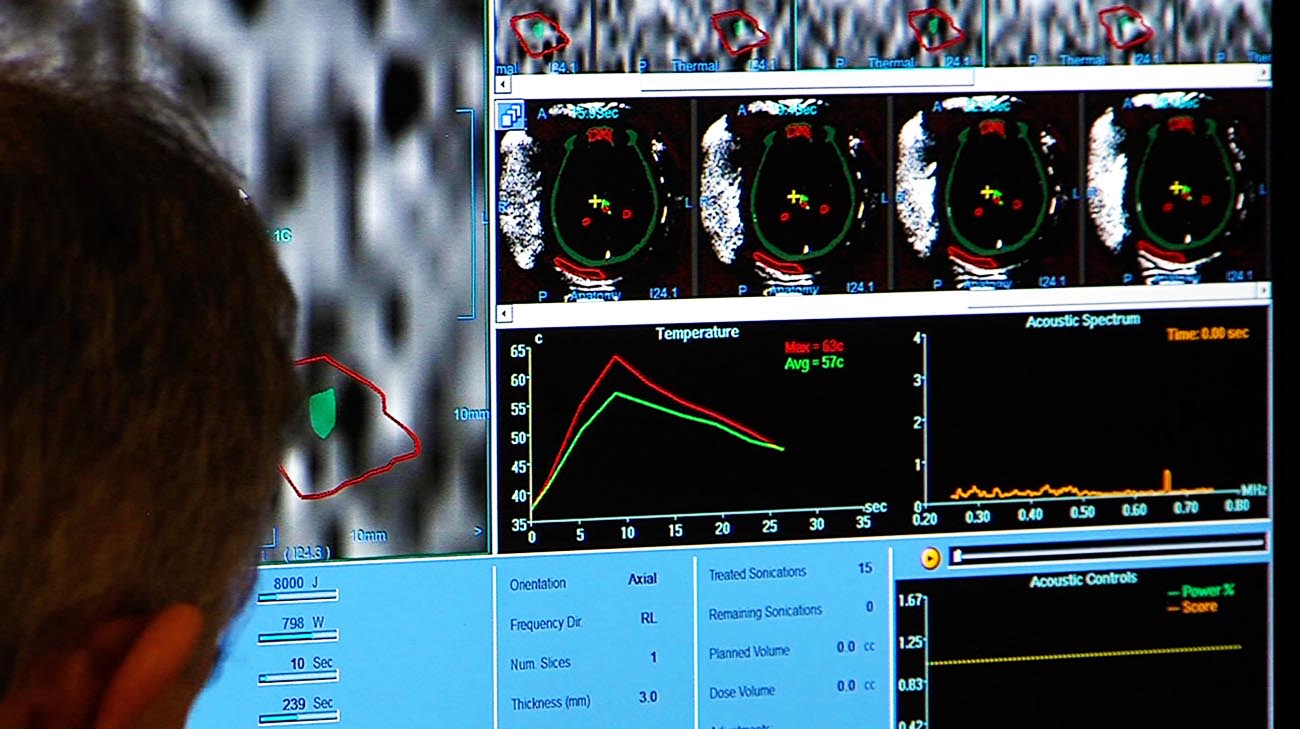
The temperature at the target rises high enough to create a tiny ablation or burn and provide a therapeutic effect, reducing the hand tremor. During the procedure ultrasound waves pass through the skull and are focused on a specific target in the brain. It is based on high intensity focused ultrasound guided by MR imaging. What is the Neuravive treatment? Neuravive is a new treatment for essential tremor where sound waves are focused through the skull to a target without the need for incisions, brain implants or radiation. Heart or spine issues that may make it difficult to tolerate the treatment or lie still for about 3 hours.Metallic implants such as a pacemaker, neurostimulator, spine or bone fixation device, total joint replacement, metal clips, screws or cochlear implants.MRI-guided focused ultrasound is not suitable for all patients. Patients with essential tremor must be at least 22 years old and patients with tremor-dominant Parkinsons disease must be at least 30 years old to receive this treatment. MRI-guided focused ultrasound may be a treatment option for people with essential tremor or with Parkinsons disease when hand tremor is the primary symptom and medications have not worked.
Focused ultrasound for essential tremor trial#
A sham-controlled trial of patients with markedly asymmetric Parkinson’s disease found that focused ultrasound subthalamotomy performed in one hemisphere resulted in improved motor scores at 4 months, but was associated with adverse events including dyskinesias and other neurologic complications. “Movement disorder neurologists now can offer their Parkinson’s patients a less invasive surgical option as part of their treatment plan,” said Paul Fishman, MD, PhD, of the University of Maryland, in a statement.Įxablate Neuro previously was approved to treat medication-refractory essential tremor and tremor-dominant Parkinson’s disease.Įarly studies suggested that focused ultrasound subthalamotomy and pallidotomy performed on one side may reduce motor manifestations of Parkinson’s disease. The device uses MRI-guided focused ultrasound waves to target and ablate the globus pallidus, requiring no incisions or brain implants.

With this new indication, Exablate Neuro is approved for unilateral pallidotomy in medication-refractory Parkinson’s patients with moderate to severe motor complications. The FDA expanded the approval of Exablate Neuro focused ultrasound to treat advanced Parkinson’s disease patients with mobility, rigidity, or dyskinesia symptoms, device maker Insightec announced Wednesday. Expanded Indication Includes Medicationīy Judy George, Senior Staff Writer, MedPage Today November 5, 2021 The procedure requires a multi-disciplinary team, including a neurosurgeon, movement disorder neurologist, and neuroradiologist. UMMC is one of only several sites in the Mid-Atlantic region with the capabilities and expertise to perform focused ultrasound for Parkinson’s disease and other movement disorders. Food and Drug Administration to treat advanced Parkinson’s disease on one side of the brain. The device, called Exablate Neuro and manufactured by Insightec, was approved in late 2021 by the U.S. Recent FDA approval of a device used in the procedure effectively opens up access to focused ultrasound beyond clinical trial participation. Focused Ultrasound for Essential Tremor and ParkinsonsĪ non-invasive ultrasound treatment for Parkinson’s disease that was tested in a pivotal trial led by University of Maryland School of Medicine researchers is now more broadly available at the University of Maryland Medical Center.


 0 kommentar(er)
0 kommentar(er)
